The majority of patients rated MIEBO as
COMFORTABLE OR VERY COMFORTABLE
on instillation*
IN GOBI AND MOJAVE PIVOTAL TRIALS1:
IN THE KALAHARI STUDY2:
The majority of patients†:

Were satisfied with MIEBO treatment

Found MIEBO comfortable

Said MIEBO was easy to administer
Most patients (~94%) were considered compliant with dosing throughout the study‡
Only ~5% of patients (n = 10) used adjunctive artificial tears/mineral oil, as permitted, after Week 4
IN THE PHASE 4 STUDY3§:

Consistently high patient satisfaction

At Day 14, median satisfaction score
increased to 90
Patient-reported treatment satisfaction


SD, standard deviation.
*Questionnaire was given on Day 1 of the GOBI and MOJAVE studies. Mean pooled comfort score was 8.0 for MIEBO and 8.4 for saline (scale of 0-10, 0 = not comfortable and 10 = very comfortable). 81% of patients treated with MIEBO reported a score of 7 or higher.1
†Mean Visual Analog Scale (VAS) score (SD) was assessed at Week 52. Respondents rated MIEBO with the following scores (scale of 0-10): satisfaction, 8.0 (2.3); comfort, 8.4 (2.1); ease of administration, 8.9 (1.9).2
‡Defined as administration of 80% to 120% of the expected doses.2
§Treatment satisfaction was rated on a VAS from 0 (extremely dissatisfied) to 100 (extremely satisfied).3
¶At the 5-minute post-dose assessment on Day 1, patients selected up to 3 terms (out of 10) to describe how MIEBO felt when placed in the eyes. The majority chose silky (68.7%), smooth (67.7%), and soothing (65.7%).3
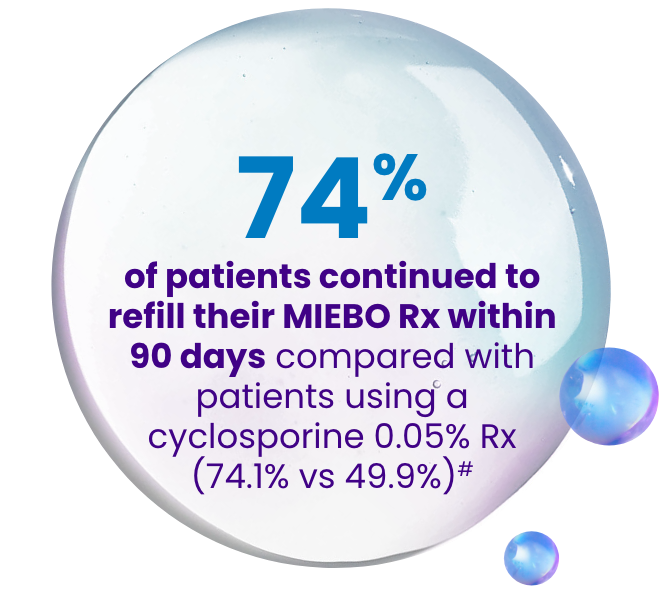
In a real-world claims database study including 7209 patients initiating MIEBO and 75,871 patients initiating cyclosporine 0.05%4:
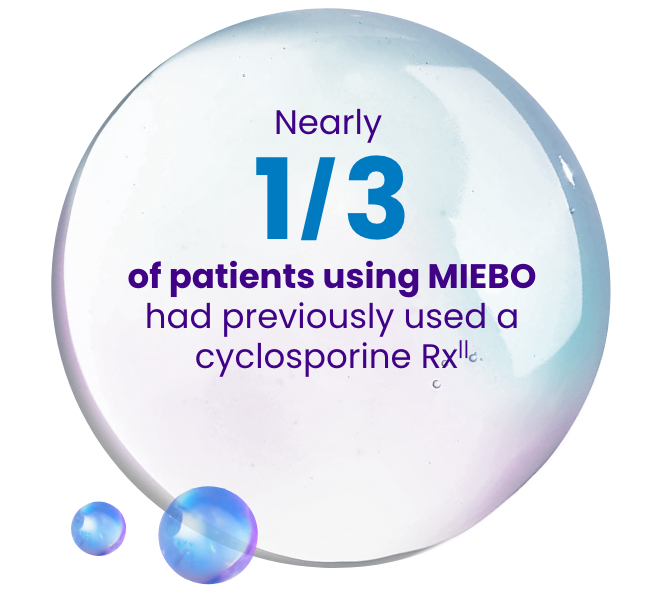
||At baseline, 21.8% of patients had taken cyclosporine 0.05% and 9.5% of patients had taken cyclosporine 0.09%.4
#Based on the secondary analysis of patients who had ≤30 days’ supply on the index date and only 1 index therapy claim on the index date.4
Study design: A retrospective database study used IQVIA’s Longitudinal Prescription Claims (LRx) and Professional Fee Claims (Dx) databases and included adult patients newly initiating perfluorohexyloctane or cyclosporine 0.05% with ≥1 claim between September 1, 2023, and November 30, 2023. Demographics, index prescription characteristics, 12-month baseline clinical characteristics, and 90-day refill rates were descriptively compared between cohorts. Initial refill rates were defined as the proportion of patients with ≥1 prescription for the index therapy within 90 days after the index date. In a secondary analysis, refill rates were also reported among the subset of patients with ≤30 days’ supply on the index date to provide a more equal comparison between the therapy cohorts.4
Study limitations include open-source claims databases utilizing data limited to contributing pharmacies/offices (Rx fill data does not necessarily indicate patient usage) and limited follow-up time. Data should be interpreted with the study design and these limitations in mind. No formal conclusions should be drawn.4
Set expectations with them by sharing the following tips:



How to
Use MIEBO

Not actual size.
One drop per eye, QID dosing5
Preservative free and provided
in a convenient multidose bottle (1-month supply)5,9
MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO.5
CORRECTLY IDENTIFY MIEBO
Ensure you get the right MIEBO when searching online or in an electronic medical/health record (EMR/EHR) system.
Remember: “i before e, to treat DED.”
MIEBO should be stored at room temperature: 15-25 °C (59-77 °F). After opening, MIEBO can be used until the expiration date on the bottle.5
QID, 4 times daily.
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full Prescribing Information for MIEBO.
References: 1. Data on file. Bausch & Lomb, Inc. 2. Protzko EE, Segal BA, Korenfeld MS, Krösser S, Vittitow JL. Long-term safety and efficacy of perfluorohexyloctane ophthalmic solution for the treatment of patients with dry eye disease: the KALAHARI study. Cornea. 2024;43(9):1100-1107. doi:10.1097/ICO.0000000000003418 3. Bacharach J, Kannarr SR, Verachtert A, et al. Early effects of perfluorohexyloctane ophthalmic solution on patient-reported outcomes in dry eye disease: a prospective, open-label, multicenter study. Ophthalmol Ther. 2025;14(4):693-704. doi:10.1007/s40123-025-01097-z 4. Shen Lee B, Pizzicato L, Langford E, et al. Early adoption and utilization of perfluorohexyloctane for dry eye disease in the United States. Clin Ophthalmol. 2025;19:2529-2540. doi:10.2147/OPTH.S529837 5. MIEBO. Prescribing Information. Bausch & Lomb, Inc. 6. Vittitow J, Kissling R, DeCory H, Borchman D. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98:100704. doi:10.1016/ j.curtheres.2023.100704 7. Agarwal P, Khun D, Krösser S, et al. Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics. Ocul Surf. 2019;17 (2):241-249. doi:10.1016/j.jtos.2019.02.010 8. Meinert H, Roy T. Semifluorinated alkanes – a new class of compounds with outstanding properties for use in ophthalmology. Eur J Ophthalmol. 2000;10 (3):189-197. doi:10.5301/EJO.2008.1838 9. Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397-1418. doi:10.1007/s40123-023-00669-1 10. Schmidl D, Bata AM, Szegedi S, et al. Influence of perfluorohexyloctane eye drops on tear film thickness in patients with mild to moderate dry eye disease: a randomized controlled clinical trial. J Ocul Pharmacol Ther. 2020;36 (3):154-161. doi:10.1089/jop.2019.0092
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full Prescribing Information for MIEBO.
FAQs